GSK’s older adult respiratory syncytial virus (RSV) vaccine candidate shows 94.1% reduction in severe RSV disease and overall vaccine efficacy of 82.6% in pivotal trial
Published By GSK [English], Thu, Oct 13, 2022 12:22 AM
For media and investors only
Data to be presented at IDWeek 2022 showed overall vaccine efficacy against RSV-lower respiratory tract disease (LRTD) in adults aged 60 years and above, with a favourable safety profile
Consistent high vaccine efficacy observed against LRTD in severe disease (94.1%), adults aged 70-79 years (93.8%) and in adults with underlying comorbidities (94.6%)
High vaccine efficacy is consistent across RSV A and B strains
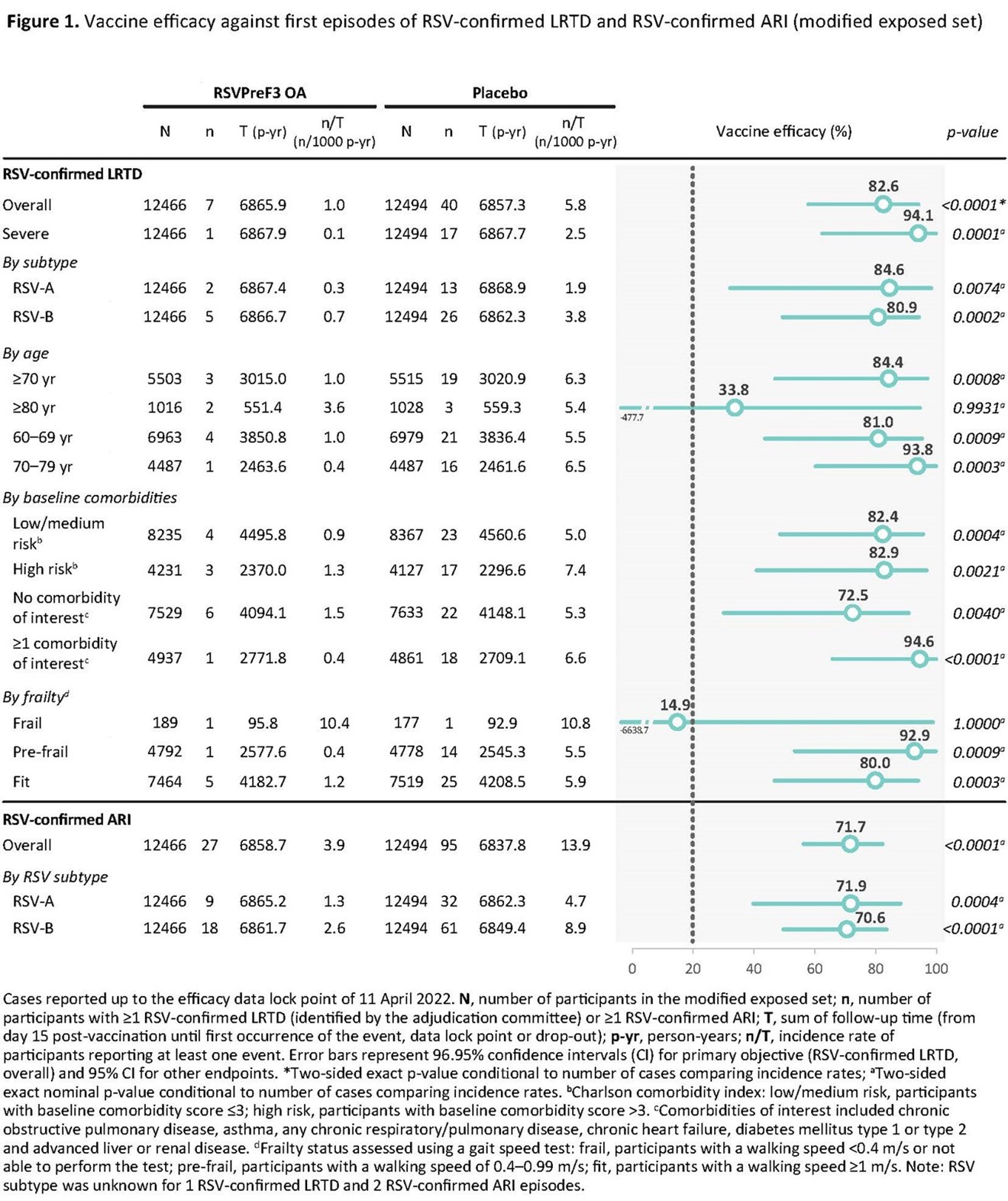
GSK plc (LSE/NYSE: GSK) today announced positive pivotal phase III trial results for its respiratory syncytial virus (RSV) vaccine candidate for adults aged 60 years and above to be presented at IDWeek 2022. The vaccine candidate was highly efficacious, demonstrating overall vaccine efficacy of 82.6% (96.95% CI, 57.9–94.1, 7 of 12,466 vs. 40 of 12,494) against RSV lower respiratory tract disease (RSV-LRTD), meeting the trial’s primary endpoint.
Consistent high vaccine efficacy was also observed across a range of pre-specified secondary endpoints, highlighting the impact the vaccine candidate could have on the populations most at risk of the severe outcomes of RSV. Efficacy against severe RSV-LRTD, defined as LRTD with at least two lower respiratory signs or assessed as severe by the investigator and confirmed by the external adjudication committee, was 94.1% (95% CI, 62.4–99.9, 1 of 12,466 vs. 17 of 12,494). In participants with pre-existing comorbidities, such as underlying cardiorespiratory and endocrinometabolic conditions, vaccine efficacy was 94.6% (95% CI, 65.9–99.9, 1 of 4,937 vs. 18 of 4,861), with 93.8% (95% CI, 60.2-99.9, 1 of 4,487 vs. 16 of 4,487) efficacy observed in adults aged 70-79 years.
Vaccine efficacy against LRTD was consistent across both RSV-A and RSV-B subtypes (84.6%; CI 32.1–98.3, 2 of 12,466 vs. 13 of 12,494 and 80.9%; CI 49.4–94.3, 5 of 12,466 vs. 26 of 12,494 respectively), consistent with the robust neutralising antibody response generated against both subtypes. See Figure 1: Vaccine efficacy against first episodes of RSV-confirmed LRTD and RSV-confirmed ARI (modified exposed set).
Tony Wood, GSK Chief Scientific Officer, said:
These are truly exceptional results given that today RSV remains one of the major infectious diseases without a vaccine, despite over 60 years of research. We believe that with the high vaccine efficacy demonstrated in this pivotal trial, our vaccine candidate has the potential to help reduce the significant global burden of RSV-associated disease in older adults, including those at the greatest risk of severe outcomes due to their age or underlying comorbidities.
The vaccine was well tolerated with a favourable safety profile. The observed solicited adverse events were typically mild-to-moderate and transient, the most frequent being injection site pain, fatigue, myalgia, and headache.
Regulatory submissions based on the phase III data are anticipated in the second half of 2022. GSK’s RSV vaccine candidate for older adults contains a recombinant subunit prefusion RSV F glycoprotein antigen (RSVPreF3) combined with GSK’s proprietary AS01E adjuvant. There are currently no RSV vaccines approved anywhere in the world.
Editor's note: This press release was originally published on 13 October 2022 and has been updated for comprehensiveness on 20 October 2022.
About the AReSVi-006 trial
The AReSVi-006 (Adult Respiratory Syncytial Virus) phase III trial is a randomised, placebo-controlled, observer-blind, multi-country trial to demonstrate the efficacy of a single dose of GSK's adjuvanted RSVPreF3 OA investigational vaccine in adults aged 60 years and above. Approximately 25,000 participants were enrolled from 17 countries.
This phase III efficacy trial is part of a comprehensive RSV evidence-generation programme conducted by GSK. It will continue to evaluate an annual revaccination schedule and longer-term protection over multiple seasons following one dose of the RSV vaccine candidate.
AReSVi-006 is closely monitored for safety, with safety data reviewed internally and by an external Independent Data Monitoring Committee on an ongoing basis.
The GSK proprietary AS01 adjuvant system contains QS-21 Stimulon® adjuvant licensed from Antigenics Inc, a wholly owned subsidiary of Agenus Inc.
About respiratory syncytial virus (RSV) in adults
RSV is a common contagious virus affecting the lungs and breathing passages. It is one of the major remaining infectious diseases for which there is currently no vaccine or specific treatment available for adults. Older adults are at high risk for severe disease due to age-related decline in immunity and underlying conditions. RSV can exacerbate conditions, including chronic obstructive pulmonary disease (COPD), asthma and chronic heart failure and can lead to severe outcomes, such as pneumonia, hospitalisation, and death. RSV causes over 420,000 hospitalisations each year and 29,000 deaths in adults in industrialised countries. Adults with underlying conditions are more likely to seek medical advice and have higher hospitalisation rates than adults without these conditions.
GSK is a global biopharma company with a purpose to unite science, technology, and talent to get ahead of disease together. Find out more at gsk.com/company.
Cautionary statement regarding forward-looking statements
GSK cautions investors that any forward-looking statements or projections made by GSK, including those made in this announcement, are subject to risks and uncertainties that may cause actual results to differ materially from those projected. Such factors include, but are not limited to, those described in the Company's Annual Report on Form 20-F for 2021, GSK’s Q2 Results for 2022 and any impacts of the COVID-19 pandemic.
Press release distributed by Wire Association on behalf of GSK, on Oct 13, 2022. For more information subscribe and follow GSK